How Nixie Tubes works
What is the Nixie Tube?
If you have a digital alarm clock or a timer on a microwave oven, VCR, or DVD player, chances are it has blue-green numbers, in which case it’s using a VFD (vacuum florescent display) or red ones, which means it’s made from LEDs (light-emitting diodes). Either way, you’ll notice that it displays each of the digit in the time (10:30 or whatever) by lighting up a pattern of seven quite separate bars, commonly called “segments”. You can write all the numbers from 0 to 9 with a seven-segment display and quite a few letters and words as well.

Nixie Tubes also display the numbers 0–9 but in a completely different way. Look closely at a Nixie Tube and you’ll see it has the ten decimal digits made out of bent wire and arranged in a stack, one in front of the other, inside a sealed glass bulb. Underneath the bulb, there are lots of electrical contacts. Wire these up to an appropriate electronic circuit and you can make the numbers count up in sequence, tell the time, or do all kinds of other neat things. Unlike VFDs and LEDs, one of the quirky things about Nixie Tubes is that different numbers light up in front of or behind one another (on different “planes”), so some digits appear brighter than others and closer to or further from your eye. You can just about see that:

How does a Nixie Tube work?
Nixie Tubes work a lot like neon lamps (although not exactly like neon lamps, as we’ll see in a moment). Why do all the metal numbers have to be sealed inside a glass bulb? Well, you can’t see just by looking, but the glass bulb is filled with a mixture of invisible gases (usually neon, mercury, and argon), and its purpose is to stop those gases from escaping (mercury is toxic so you don’t want that floating around anywhere near you). The bent metal wires that display each number aren’t filaments, like the ones in an incandescent lamp. Each of them works as a separate negative terminal (cathode) in what is effectively a gas-discharge tube — so one Nixie Tube has ten cathodes. The cathodes don’t actually touch one another but are kept apart by tiny ceramic spacers. There’s also a single positive terminal (anode), shaped like a mesh or grid, that’s wrapped around the stack of numeric cathodes and serves all 10 of them.

Different kinds of lamps make light in completely different ways. Old-style electric lights and flashlights (torches) use incandescent lamps, which glow because a metal filament inside them gets hot when electricity flows through it. If those lamps glow red hot, fluorescent lamps glow “white cold”: they turn electricity into invisible ultraviolet light, which gets converted into visible light by the white coating that lines the inside of their tubes. Neon lamps are similar to fluorescent lamps except that they make visible (red) light directly. When you switch on the power, atoms of neon gas are split apart inside the tube to make electrons and ions, which collide, and give off red-colored light.

Nixie Tubes are very similar to neon lamps. Both look a bit like cathode-ray tubes (old-style televisions), in which electrons boil off a hot metal cathode at one end and race down the Tube toward a positively charged anode at the other. But in neon lamps and Nixies, the cathodes remain relatively cool (tubes like this are described as “cold cathode,” even though they’re generally on the warm side — about human body temperature) but the gas mixture that surrounds them is at a very low pressure (perhaps 1/100th of normal atmospheric pressure or even less — so typically less than 1000Pa or 0.01 atmospheres). When a voltage of about 170–180 volts is applied between the anode and one of the cathodes, the low-pressure gas becomes ionized (its atoms or molecules are turned into positively charged ions and negatively charged electrons). When electrons, ions and atoms collide with one another (and with metal atoms ejected or “sputtered” from the cathode), we get a glowing, fuzzy “coating” of light forming all around the cathode, very close to it, that follows its shape precisely — effectively making it appear as though one of the numbers 0–9 is illuminated. Applying a voltage to a different cathode makes a different digit “light up.”

What makes that ghostly glow?
In an incandescent lamp the coiled filament glows because it’s red or white hot. But in a Nixie Tube something very different is going on: first of all the cathode is cold so the glow isn’t being produced by heat; second, the glow happens some distance beyond the cathode that’s producing it — it’s a kind of “ghost of a glow” some way removed from the cathode itself. What we see here is called a cold-cathode glow discharge. Why does it happen… and why is it some distance away from the cathode?

When a high-enough voltage is applied between the wire-mesh anode and one of the numeric cathodes, the molecules or atoms of the low-pressure gas inside the glass tube split into positively charged ions and negatively charged electrons, making a sort of “soup” of hot plasma. The positive ions are pulled toward the negatively charged cathode (the wire-outline number), while the negative electrons head for the positively charged anode mesh. When the ions hit the cathode, they knock further (secondary) electrons out of it, which also head out into the plasma. It’s this dual movement of charged particles that allows an electric current to flow through the tube. Much of the light that glows from a Nixie Tube is produced by collisions between gas atoms, ions and electrons, just like in a neon tube. But some of it is also produced another way.

Some of the positive ions hit the cathode directly, while others hit the gas atoms and push those into the cathode instead. Like tiny atomic bullets fired at a wall, these small gas atoms and ions chip bigger metal atoms out of the cathode so they’re ejected into the main part of the tube — a process known as sputtering. These sputtered atoms then undergo collisions of their own in the plasma, absorbing energy to become “excited” and unstable, then losing their energy again by giving off photons of light and contributing to the overall glow.
So, in short, the glow inside a Nixie Tube is produced by a combination of ionization and sputtering.
Glow discharges like this are complex and, in some tubes, produce a whole series of light and dark bands between the anode and the cathode. Nixie Tubes are carefully designed so that all we can really see is a single glow surrounding the cathode. But if you’re observant, you’ll also see that there’s a thin, completely dark area between the glow and the cathode (called the Aston dark space). Beyond that is the ghostly “cathode glow” we see forming the lit-up number. So why the space between the cathode and the glow appears? The glow happens when electrons, fired out from the cathode and traveling toward the anode, collide with atoms and ions in the main part of the tube. The electrons accelerate from the cathode to the anode, picking up speed and energy as they go. When they’re very near the cathode, the electrons vastly outnumber the ions and atoms there and they don’t have much energy. So the chance of a collision between an electron and an atom is relatively small and, even if it does happen, the electron doesn’t have enough energy to excite the atom into giving off light. That’s why this area is dark. A bit further away from the cathode, the electrons have gained more speed and more energy, and there are more atoms for them to collide with. When electrons collide with atoms and ions in this region, they can excite them enough to make them give off photons of visible light — hence the “cathode glow.”
In summary
Let’s quickly summarize all described with a small diagram:
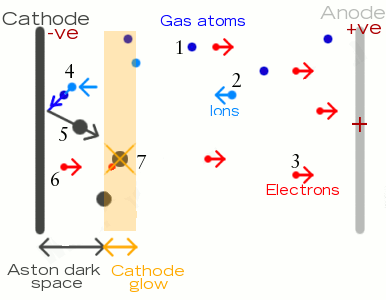
- Gas atoms in the tube are at low-pressure. When a high enough voltage (“ionization potential”) is applied between the anode and cathode, the atoms split into a plasma of ions and electrons
- The positively charged ions are attracted toward the negatively charged cathode. When they hit the cathode, some of them knock out energetic
- These ejected, negatively charged electrons (known as secondary electrons) are attracted toward the positively charged anode. Collisions between these electrons and the ions they encounter produce most of the light in the tube, just like in a neon lamp
- Near the cathode, ions push gas atoms into the cathode itself
- Metal atoms are ejected (“sputtered”) from the surface of the cathode
- Electrons leaving the cathode are pulled toward the positively charged anode. Close to the cathode, there are more electrons than ions but the electrons have relatively low speed and energy. Collisions between electrons and atoms or ions don’t excite them enough to produce light, so this area is dark (the Aston dark space)
- At a certain distance from the cathode, electrons have picked up more speed and energy. When they collide with atoms or ions here, they produce photons of visible light — giving that familiar glow just beyond the cathode.

